CROSSLINKING HYALURONIC ACID WITH LIDOCAINE
FULL LINKED MATRIX TECHNOLOGY
DermiKo. The crosslinked Hyaluronic Acid based filler to reduce the visible signs of aging.

EASY
DermiKo is elastic and very easy to dose, offering straightforward placement and harmonious results.

ACCURATE
Pharmaceutical grade disposable sterile syringes made of glass to preserve the product in
any condition. Ergonomic grip system consisting of plunger and shaped flange made of highly compact polycarbonate. Latest generation technical needles for easy and precise extrusions.

SAFE
All products of the DermiKo line have an extremely low crosslinking agent residue thanks
to the low temperature, high vacuum, crosslinking system developed by Bioitech, enabling the
use of lower BDDE concentrations before reaction compared to other products, and a final
certified residue of less than 0.1 PPM.
DERMIKO PRODUCTS ARE MANUFACTURED
• based on hyaluronic acid from biofermentation;
• with high pharmaceutical-grade raw materials;
• in a controlled environment with the latest generation tools and equipment.
TRACKING
Each product is identified by a specific batch number, present on the clear traceability label on the syringe, ready to be peeled off and applied to the patient’s medical record.
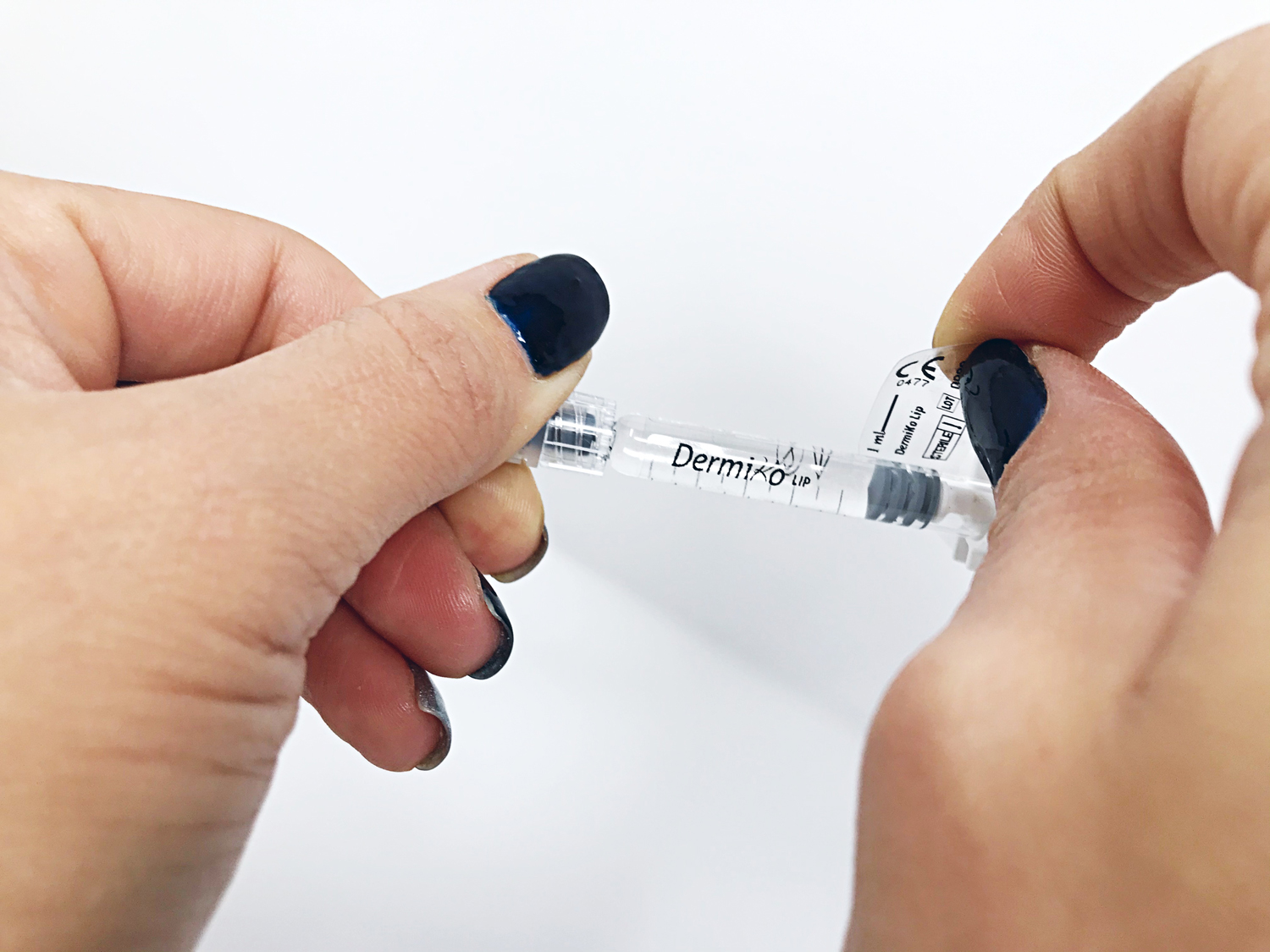
COMFORT
The products of the DermiKo line contain Lidocaine for greater patient comfort during administration. The shelf life of the products of the DermiKo line with Lidocaine is equal to or greater than that of market products without Lidocaine.
FULL LINKED MATRIX TECHNOLOGY
100% cross linked gel without phosphate buffer dilution in the final phase
• Greater treatment effectiveness thanks to the complete cross-linking of the gel which is not diluted in the final phase.
• Lidocaine inclusion, with a more homogeneous distribution within the product.
• Reduction of post-application swellings.
KNOW-HOW and TECHNOLOGY
Bioitech’s Aesthetic Line are the only products that incorporate the know-how of crosslinking methods developed for products in other areas of medicine and on different raw materials. The hyaluronic acid line developed by Bioitech integrates three proprietary technologies:
• High Vacuum Technology
• Full Matrix link Technology
• Soft flow Technology
DERMIKO IS A FOURTH GENERATION FILLER
BASED ON HYALURONIC ACID
First generation: monophasic and / or biphasic gels with fleeting results; uneven and hard to inject.
Second generation: improved injection (industrial filtration systems) and comfort (addition of lidocaine)
Third generation: improved rheology by optimizing crosslinking methods and the concentration of hyaluronic acid; included “additive” substances such as mannitol; improved residual BDDE which usually range under < 0.5 ppm.
DermiKo fourth generation:
• Improved rheological performance with Tangents (TAN) below 0.1.
• Reduced BDDE residue to certified values <0.1 PPM thanks to low temperature Crosslinking technologies.
• Improved syringe shape for better use and latest technology needles for a fluid infiltration, even with 30G ½ needles.
DermiKo is a Class III sec. Directive 93/42/EEC medical device.
DermiKo is CE0477 certified.
DermiKo is certified for intradermal and subcutaneous filling.
INSTRUCTION FOR USE
In compliance with current legislation, I declare under my responsibility that I am a professional in the medical sector and that I am therefore authorized to view the content on this web page

